In
Planetary Greenhouse we considered an atmosphere transparent to some
long-wave radiation and opaque to others. In Circulating Cells Version 8.2, which you can download
here, we simulate such an atmosphere with the new
transparency fraction parameter.
For the moment, we ignore the temperature difference that must exist between the planet surface and the lower atmosphere (see
Surface Cooling, Part VI). We assume that the solid or liquid surface beneath one of the bottom gas cells will be at the same temperature as the gas itself. Ever since our
Earth Radiator post, we have assumed that the surface of a planet is a
black body radiator. If a bottom cell is at 300 K, the surface below will be at 300 K also, and by
Stefan's Law it will radiate 460 W/m
2. At 303 K, the radiated power increases to 480 W/m
2. Black-body radiation increases as the fourth power of the temperature, so a 1% increase in temperature causes a 4% increase in radiated power.
The absorption spectrum of the Earth's atmospheric layers varies in a complex and dramatic way with wavelength, as you can see
here. What made our
Total Escaping Power calculation so complicated was that we had to deal with the partial absorption of some wavelengths by each atmospheric layer, and therefore the partial emission of these same wavelengths by the same atmospheric layers. We want to avoid the complexity of partial absorption at a given wavelength, so we assume that the gas in our
CC8 simulation has a spectrum that vacillates between perfect transparency to perfect opacity, and does so every fraction of a micron on the wavelength scale. As a result of this vacillation, a gas cell will absorb none of the radiation at a particular wavelength, or all of it. When we look at the fraction of black-body radiation that passes through a cell, this fraction is a constant property of the cell, regardless of its temperature or pressure. We call it the
transparency fraction in the
CC8 program, and here we will call it τ.
By the principle of
radiative symmetry, each gas cells will radiate heat at the same wavelengths it absorbs. But because all the cells around it have the same absorption spectrum, none of the heat radiated by a cell will escape into space, except for the heat radiated by the cells in the top row, which we call our
tropopause. These cells radiate directly into space. The power they radiate is the power that a black body would radiate, multiplied by 1−τ.
The incoming heat from the Sun, meanwhile, passes straight through our atmosphere, because we assume that the gas is perfectly transparent to
short-wave radiation. The Sun's heat warms the planet surface, which for the moment we assume is something like sand. The sand heats up rapidly until it is losing heat by radiation and convection at the same rate it is gaining heat by absorption of the Sun's light. Some heat it radiates directly into space. The rest passes into the lower atmosphere and must be transported up to the tropopause by convection, where it is radiated into space.
Bottom Gas Cell Temperature =
TB
Planet Surface Temperature =
TS =
TB
Stefan's Constant = σ = 5.7 × 10
−8 W/m
2/K
4
Emitted Surface Radiation = σ(
TS)
4
Escaping Surface Radiation = τσ(
TS)
4
Top Gas Cell Temperature =
TT
Emitted Tropopause Radiation = σ(1-τ)(
TT)
4
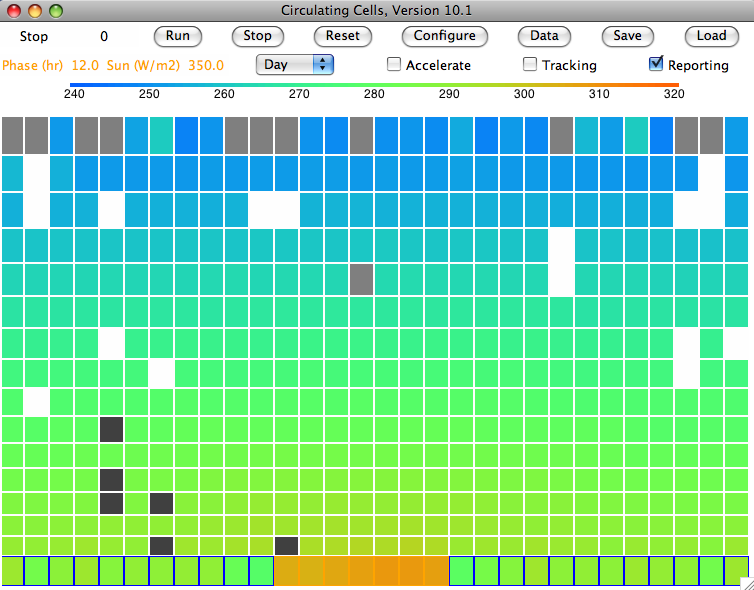
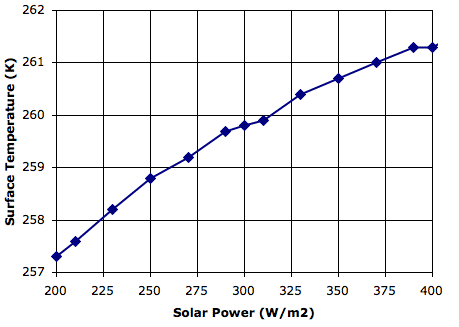
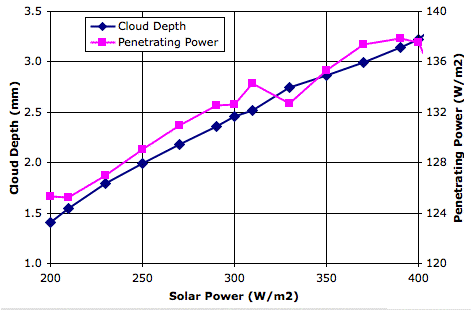
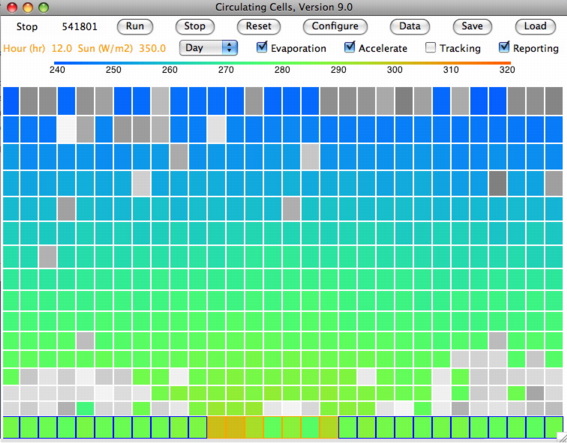
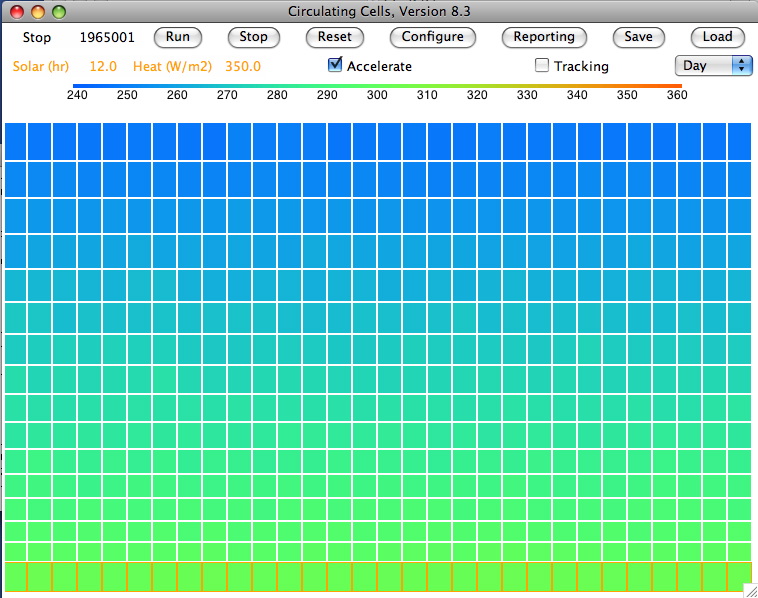
We set τ=0.50 and ran
CC8 with
Day heating and the Sun's power set to 350 W/m
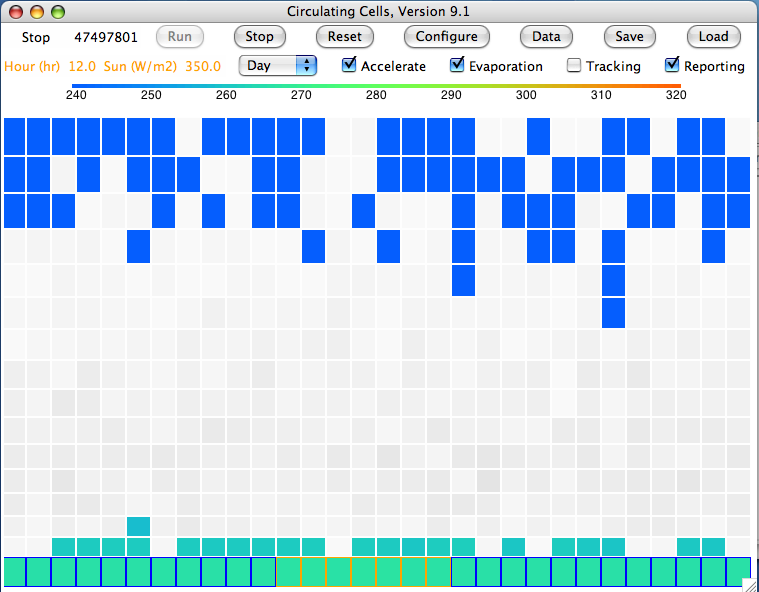
2. After ten million iterations we are confident that we have reached equilibrium, and we obtain
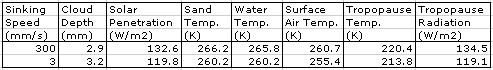
this array. The average temperature of the bottom cells is 301.1 K (28°C) and of the top cells is 252.1 K (−21°C). The heat radiated by the surface is 468.5 W/m
2, of which 234.2 W/m
2 escapes directly into space. That leaves 115.7 W/m
2 of the Sun's heat to be transported up through the atmosphere. The heat radiated by the tropopause is 115.1 W/m
2, leaving 0.7 W/m
2 unaccounted for, which is well within the range of our rounding errors and the random fluctuations in our cell temperatures.
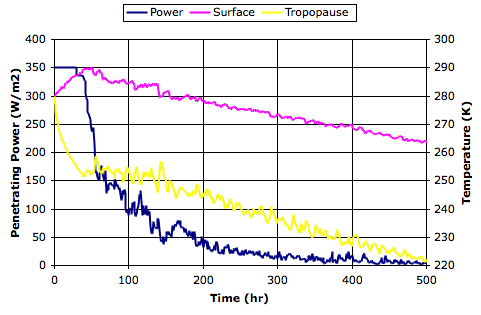
In our
previous post, we ran our simulation for an opaque atmosphere, which corresponds to τ=0.00, and the surface temperature rose to 355 K (59°C). We see that τ=0.50 allows the surface to cool by 31°C to 28°C. In
With 660 ppm CO2, we showed that doubling the CO2 concentration in the Earth's atmosphere will cause a 2% drop in the
total escaping power. So now we set τ=0.49, so as to cause a 2% drop in the power escaping directly from our simulated planet surface into space. We arrive at a
this array, in which the surface has warmed by 0.9°C to 302.0 K and the tropopause has warmed by 0.4°C to 252.5 K.
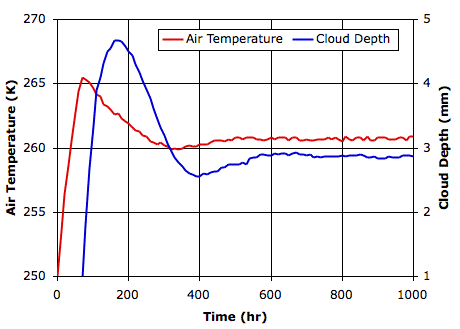
As a check, we run with τ=1.00, in which case the atmosphere is perfectly transparent and the surface radiates all its heat directly into space. The surface cools to 280 K (7°C). The atmosphere assumes the dry adiabatic lapse profile. But now we turn on the cell mixing by setting the mixing fraction to 0.2, and after a few hundred thousand iterations we see the entire atmosphere warm up to 280 K. This is the warm atmosphere we described in our original
Greenhouse Effect post and again in
Adiabatic Magic. When the atmosphere is not transporting heat to the tropopause, there is no greenhouse effect, and the atmosphere mixes until it arrives at a uniform temperature equal to the surface temperature.
We see that
CC8 can simulate the effect of changing the concentration of a greenhouse gas like CO2, simply by making small changes to its transparency fraction. Once we have included evaporation, clouds, and rain into our simulation, we will be able to estimate the effect of changes in CO2 upon the average temperature of our planet surface.