The theory of Anthropogenic Global Warming, so far as we understand it, consists of the following two assertions.
(1) If we increase the concentration of CO2 in the atmosphere to 600 ppmv, we will cause the world to warm up by at least 2°C. (The concentration in pre-industrial times was 300 ppmv and is currently 400 ppmv, where ppmv is parts per million by volume.)
(2) If we continue burning fossil fuels at our current rate, emitting 10 petagrams of carbon into the atmosphere every year, we will raise the concentration of CO2 in the atmosphere to 600 ppmv within the next one hundred years.
We can falsify the second assertion using our observations of the carbon-14. We present a detailed analysis of atmospheric carbon-14 in a series of posts starting with Carbon-14: Origins and Reservoir. Here we present a summary, with approximate numerical values that are easy to remember.
Each year, cosmic rays create 8 kg of carbon-14 in the upper atmosphere. If carbon-14 were a stable atom, all carbon in the Earth's atmosphere would be carbon-14. But carbon-14 is not stable. One in eight thousand carbon-14 atoms decays each year. The rate at which the Earth's inventory of carbon-14 decays must be equal to the rate at which it is created. There must be 64,000 kg of carbon-14 on Earth.
The Earth's atmosphere contains 800 Pg of carbon (1 Pg = 1 Petagram = 1012 kg) bound up in gaseous CO2. One part per trillion of this carbon is carbon-14 (1 ppt = 1 part in 1012). There are 800 kg of carbon-14 in the atmosphere. That leaves 63,200 kg of the total inventory somewhere else. We'll call this "somewhere else" the carbon-14 reservoir.
Each year, 8 kg of carbon-14 is created in the atmosphere by cosmic rays, and each year the atmosphere loses 8 kg of carbon-14 to the reservoir. (Here we are ignoring the 0.1 kg of atmospheric carbon-14 that decays each year.) There is no chemical reaction that can separate carbon-14 from normal carbon. Every 1 kg of carbon-14 that leaves the atmosphere for the reservoir will be accompanied by 1 Pg of normal carbon.
Consider the atmosphere before we began to add 10 Pg of carbon to it each year. The mass of carbon in the atmosphere is constant. If 1 Pg of carbon leaves the atmosphere and enters the reservoir, 1 Pg of carbon must go in the opposite direction, leaving the reservoir and entering the atmosphere.
The only way for there to be a net loss of carbon-14 from the atmosphere to the reservoir is if the concentration of carbon-14 in the reservoir is lower than in the atmosphere. The only place on Earth that is capable of acting as the reservoir is the deep ocean, in which the concentration of carbon-14 is 80% of the concentration in the atmosphere. Each year 40 Pg of carbon leaves the atmosphere and enters the deep ocean, carrying with it 40 kg of carbon-14, while 40 Pg of carbon leaves the ocean and enters the atmosphere, carrying with it 32 kg of carbon-14. The result is a net flow of 8 kg/yr of carbon-14 into the ocean. Furthermore, the ocean contains 63,200 kg of carbon-14 in concentration 0.8 ppt, so the total mass of carbon in the oceans is roughly 80,000 Pg.
With the ocean and the atmosphere in equilibrium, 40 Pg of carbon is absorbed by the ocean each year, and 40 kg is released by the ocean. If we were to double the quantity of carbon in the atmosphere, we would double the amount absorbed by the ocean each year. Instead of 40 Pg being absorbed each year, 80 Pg would be absorbed. We could double the concentration of carbon in the atmosphere by emitting 40 Pg/yr. But we emit only 10 Pg/yr. Our emissions are sufficient to increase the mass of carbon in the atmosphere by 25%, after which everything we emit will be absorbed by the oceans. The oceans contain 80,000 Pg of carbon. If we add 10 Pg/yr, it will take roughly eight thousand years to double the carbon concentration in the oceans, after which the concentration in the atmosphere will double also.
Back in the 1960s, atmospheric nuclear bomb tests doubled the concentration of carbon-14 in the atmosphere. Such tests stopped in 1967. In our more precise calculation we predict that the concentration of carbon-14 must relax after 1967 with a time constant of 17 years, so that it would be 1.37 ppt in 1984 and 1.05 ppt in 2018. The concentration did relax afterwards, with a time constant of roughly 15 years, and in 2016, the carbon-14 concentration in the atmosphere is indistinguishable from its value before the bomb tests. During that time, almost every CO2 molecule that existed in the atmosphere in 1967 passed into the ocean and was replaced by another from the ocean. Anyone claiming that our carbon emissions will remain in the atmosphere for thousands of years, such as the author of this article, is wrong. If we stopped burning fossil fuels tomorrow, the CO2 concentration of the atmosphere would return to its pre-industrial value within fifty years.
When carbon is absorbed or emitted by the ocean, it does so as a molecule of CO2. Statistical mechanics dictates that the rate of absorption is weakly dependent upon temperature, but the rate of emission is strongly dependent upon temperature. When we calculate the effect of temperature upon the equilibrium between the ocean and the atmosphere, we conclude that a 1°C warming of the oceans will cause a 10 ppmv increase in the concentration of CO2 in the atmosphere. When we look back at the record of CO2 concentration and temperature over the past 400,000 years, we see the correlation we expect, with the magnitude of the changes in good agreement with our prediction. For a 12°C increase in temperature, for example, the concentration of CO2 increases by 110 ppmv.
If we consider the atmosphere of the Earth in pre-industrial times, its atmospheric CO2 concentration was roughly 300 ppmv. A more exact value for the creation of carbon-14 is 7.5 kg/yr and we conclude that 37 Pg/yr or carbon was being absorbed and emitted by the ocean. When we add 10 Pg/yr human emissions from burning fossil fuels, we expect the concentration of CO2 in the atmosphere to rise by 27% to 380 ppmv, which is close to the 400 ppmv we observe.
Our analysis of the carbon cycle makes three independent and unambiguous predictions all of which turn out to be correct to within ±10%. Our analysis is reliable, and it tells us that it will take roughly eight thousand years to double the CO2 concentration of the atmosphere if we continue burning fossil fuels at our current rate. Assertion (2) above is wrong by two orders of magnitude. The theory of Anthropogenic Global Warming, as stated above, is untrue.
POST SCRIPT: Assertion (1) is harder to falsify, and we do not claim to have done so in a manner convincing to all readers. Nevertheless, we did conclude that assertion (1) had to be wrong in our series of posts on the greenhouse effect, which we summarize in Anthropogenic Global Warming. We calculated that doubling the CO2 concentration of the atmosphere will cause the Earth to warm up by 1.5°C, provided we ignore changes in water vapor and cloud cover. As the world warms up, however, water evaporates more quickly from the oceans, and we get more clouds. Clouds reflect sunlight. The warming effect of doubling CO2 concentration is reduced by an increase in cloud cover. Clouds stabilize the Earth's temperature because they become more frequent as the Earth warms up, and less frequent as it cools down. Our simulation of the atmosphere with clouds suggests that the actual warming caused by a doubling of CO2 will be 0.9°C. So far as we can tell, the climate models used by the majority of climate scientists do not account for the increase in cloud cover that occurs as the world warms up. But they do account for the increase in water vapor in the atmosphere. Clouds cool the world, but water vapor is another greenhouse gas, and warms the world. By including water vapor but excluding the increasing cloud cover, these climate models conclude that the effect of doubling CO2 concentration will be 2°C or larger.
POST POST SCRIPT: Some readers suggest that the atmosphere-ocean system cannot be modeled with linear diffusion because the dissolved CO2 does not increase in proportion to atmospheric CO2 concentration. We address and reject their claim in an update to Probability of Exchange. They claim that Henry's Law does not apply to CO2 and seawater, despite many measurements to the contrary, such as Tsui et al., and general acceptance of Henry's Law in academic texts such as this.
Sunday, October 9, 2016
Thursday, January 14, 2016
Carbon Cycle: The Correlation Between Temperature and CO2
In our previous post we deduced from first principles a relationship between temperature and CO2 concentration in the atmosphere of our carbon cycle. In the appendices of this post, we show that our calculation is consistent with the van t'Hoff equation used by chemists to predict the concentration of CO2 above a water reservoir, and to measurements of the solubility of CO2 in water. In the main body of this post, we will see how our calculation compares to measurements of temperature and CO2 concentration in the Earth's atmosphere over the past half-million years.
In our carbon cycle equations, MR is the mass of carbon in the oceanic reservoir, MA is the mass of carbon in the atmosphere, kR is the fraction of oceanic reservoir CO2 molecules that will be emitted into the atmosphere each year, and kA is the fraction of atmospheric CO2 molecules that will be absorbed by the oceanic reservoir each year. The rate at which carbon is emitted by the oceanic reservoir is kRMR, and the rate at which it is absorbed by the oceanic reservoir is kAMA. At equilibrium, these two rates will be the same, so we have:
kRMR = kAMA ⇒ MA = kRMR/kA (Eq. 1)
In our previous post we concluded that kA is constant with temperature, while kR increases with temperature as e−2300/T. Our consideration of the Earth's carbon-14 inventory showed that MR = 77,000 Pg. In our natural, equilibrium carbon cycle, we found that MA = 650 Pg. Given that the carbon must reside somewhere, even if MA doubles or halves with temperature, MR will change by less than 1%. So we can assume, to the first approximation, that MR is constant with temperature. It is MA that varies with temperature, and it does so in proportion to kR.
MA = kRMR/kA ∝ e−2300/T (Eq.2)
Aa two temperatures T1 and T2, the equilibrium values of MA, which we denote MA(T1) and MA(T2), are related by:
MA(T2) / MA(T1) = e−2300/T2 / e−2300/T1 = e2300(1/T1−1/T2) (Eq. 3)
Our (Eq. 3) predicts a close, positive correlation between temperature and atmospheric CO2 concentration, and it gives us an estimate of the magnitude of the change in CO2 concentration with temperature. The graph below shows atmospheric CO2 concentration provided by Barnola et al. and global temperature relative to today provided by Petit et al. over the past 425,000 years as determined from the Vostok ice cores.
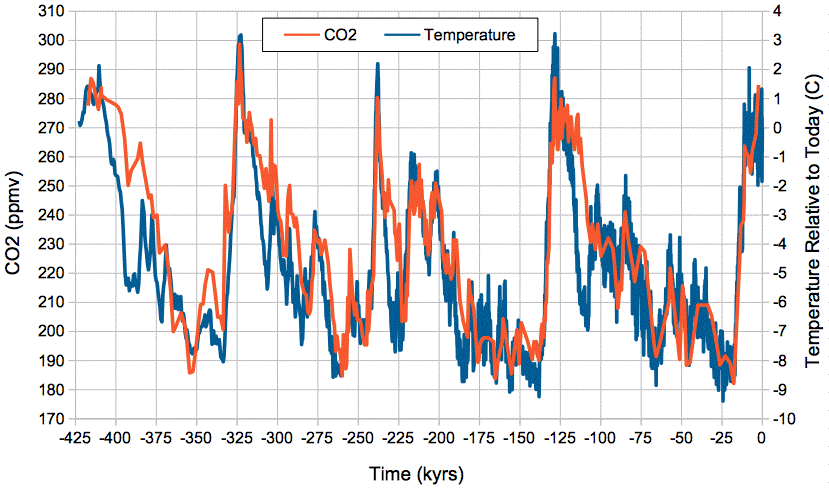
Figure: Absolute Atmospheric CO2 Concentration and Relative Temperature versus Time from Vostok ice cores. Click to enlarge. Local copies of data here and here.
We see close and sustained correlation between CO2 concentration and temperature, even as temperature varies by 12°C. If we assume today's average global temperature is 14°C = 287 K, the change from −9°C to +3°C relative to today is a swing from 278 K to 290 K. Our (Eq. 3) predicts an increase in the mass of carbon in the atmosphere by a factor of e2300(1/278−1/290) = 1.41. Because almost all carbon in the atmosphere is bound up in CO2 molecules, the concentration of CO2 in the atmosphere is proportional to the total mass of carbon in the atmosphere, so when the total mass increases by a factor of 1.41, the CO2 concentration should increase by a factor of 1.41 also. Looking at the graph, we see CO2 rising from 190 ppmv to 290 ppmv, which is a factor of 1.52. Given the many uncertainties in our calculations, and in the ice-core measurements themselves, we are well-satisfied with the agreement between our calculations and the magnitude of the CO2 concentration changes in the ice core measurements.
We conclude that the correlation between CO2 concentration and temperature in the Earth's atmosphere over the past half million years is due to the effect of temperature upon the exchange of CO2 between the atmosphere and the oceanic reservoir of the Earth's carbon cycle. As the temperature rises, the CO2 concentration rises, and when temperature falls, the CO2 concentration falls.
Appendix 1: The van t'Hoff equation for CO2 and water states that the concentration of CO2 above the water is proportional to e−2400/T. Our (Eq. 2) states that it is proportional to e−2300/T. We are well-satisfied with this agreement.
Appendix 2: Suppose we have pure CO2 gas at atmospheric pressure above a reservoir of water. No matter how much CO2 the water dissolves, we maintain the same pressure of CO2 above the water. In this arrangement, unlike the arrangement of our carbon cycle, the concentration of CO2 in the water can vary. Applying the same reasoning we presented in our previous post, the rate at which CO2 molecules are absorbed by the water remains constant with temperature. At equilibrium, the rate at which CO2 molecules are emitted by the water must equal the rate at which they are absorbed, which means the rate of emission must also remain constant. But our calculation states that the probability of any given CO2 molecule in the water being emitted in a certain interval of time must increase with temperature as e−2300/T. If the rate of emission is to remain constant, the concentration of CO2 in the water must decrease with temperature as e2300/T. Only then will the rate of CO2 emission by the water, which is the product of the probability and the concentration, remain constant with temperature. We examine the plot of CO2 solubility in water versus temperature here. As temperature increases from 10°C to 20°C, the solubility of CO2 in water drops from 2.5 g/kg to 1.25 g/kg, which is a factor of 0.50. Our calculation suggests that it should drop by a factor of 0.58. We are well-satisfied with this agreement.
In our carbon cycle equations, MR is the mass of carbon in the oceanic reservoir, MA is the mass of carbon in the atmosphere, kR is the fraction of oceanic reservoir CO2 molecules that will be emitted into the atmosphere each year, and kA is the fraction of atmospheric CO2 molecules that will be absorbed by the oceanic reservoir each year. The rate at which carbon is emitted by the oceanic reservoir is kRMR, and the rate at which it is absorbed by the oceanic reservoir is kAMA. At equilibrium, these two rates will be the same, so we have:
kRMR = kAMA ⇒ MA = kRMR/kA (Eq. 1)
In our previous post we concluded that kA is constant with temperature, while kR increases with temperature as e−2300/T. Our consideration of the Earth's carbon-14 inventory showed that MR = 77,000 Pg. In our natural, equilibrium carbon cycle, we found that MA = 650 Pg. Given that the carbon must reside somewhere, even if MA doubles or halves with temperature, MR will change by less than 1%. So we can assume, to the first approximation, that MR is constant with temperature. It is MA that varies with temperature, and it does so in proportion to kR.
MA = kRMR/kA ∝ e−2300/T (Eq.2)
Aa two temperatures T1 and T2, the equilibrium values of MA, which we denote MA(T1) and MA(T2), are related by:
MA(T2) / MA(T1) = e−2300/T2 / e−2300/T1 = e2300(1/T1−1/T2) (Eq. 3)
Our (Eq. 3) predicts a close, positive correlation between temperature and atmospheric CO2 concentration, and it gives us an estimate of the magnitude of the change in CO2 concentration with temperature. The graph below shows atmospheric CO2 concentration provided by Barnola et al. and global temperature relative to today provided by Petit et al. over the past 425,000 years as determined from the Vostok ice cores.

Figure: Absolute Atmospheric CO2 Concentration and Relative Temperature versus Time from Vostok ice cores. Click to enlarge. Local copies of data here and here.
We see close and sustained correlation between CO2 concentration and temperature, even as temperature varies by 12°C. If we assume today's average global temperature is 14°C = 287 K, the change from −9°C to +3°C relative to today is a swing from 278 K to 290 K. Our (Eq. 3) predicts an increase in the mass of carbon in the atmosphere by a factor of e2300(1/278−1/290) = 1.41. Because almost all carbon in the atmosphere is bound up in CO2 molecules, the concentration of CO2 in the atmosphere is proportional to the total mass of carbon in the atmosphere, so when the total mass increases by a factor of 1.41, the CO2 concentration should increase by a factor of 1.41 also. Looking at the graph, we see CO2 rising from 190 ppmv to 290 ppmv, which is a factor of 1.52. Given the many uncertainties in our calculations, and in the ice-core measurements themselves, we are well-satisfied with the agreement between our calculations and the magnitude of the CO2 concentration changes in the ice core measurements.
We conclude that the correlation between CO2 concentration and temperature in the Earth's atmosphere over the past half million years is due to the effect of temperature upon the exchange of CO2 between the atmosphere and the oceanic reservoir of the Earth's carbon cycle. As the temperature rises, the CO2 concentration rises, and when temperature falls, the CO2 concentration falls.
Appendix 1: The van t'Hoff equation for CO2 and water states that the concentration of CO2 above the water is proportional to e−2400/T. Our (Eq. 2) states that it is proportional to e−2300/T. We are well-satisfied with this agreement.
Appendix 2: Suppose we have pure CO2 gas at atmospheric pressure above a reservoir of water. No matter how much CO2 the water dissolves, we maintain the same pressure of CO2 above the water. In this arrangement, unlike the arrangement of our carbon cycle, the concentration of CO2 in the water can vary. Applying the same reasoning we presented in our previous post, the rate at which CO2 molecules are absorbed by the water remains constant with temperature. At equilibrium, the rate at which CO2 molecules are emitted by the water must equal the rate at which they are absorbed, which means the rate of emission must also remain constant. But our calculation states that the probability of any given CO2 molecule in the water being emitted in a certain interval of time must increase with temperature as e−2300/T. If the rate of emission is to remain constant, the concentration of CO2 in the water must decrease with temperature as e2300/T. Only then will the rate of CO2 emission by the water, which is the product of the probability and the concentration, remain constant with temperature. We examine the plot of CO2 solubility in water versus temperature here. As temperature increases from 10°C to 20°C, the solubility of CO2 in water drops from 2.5 g/kg to 1.25 g/kg, which is a factor of 0.50. Our calculation suggests that it should drop by a factor of 0.58. We are well-satisfied with this agreement.
Tuesday, January 5, 2016
Carbon Cycle: Effect of Temperature
Up to now, we assumed that the temperature of our carbon cycle remained constant. We never specified what the temperature of the ocean or the atmosphere was. Nor did we assume that the temperature of the ocean or the atmosphere was uniform. We merely assumed that the temperature in each part of the system remained constant. Today we estimate how the rate of transfer coefficients of our carbon cycle, kA and kR, will change with temperature. We recall that kA is the fraction of atmospheric CO2 molecules that will be absorbed by the oceanic reservoir each year, and kR is the fraction of oceanic reservoir CO2 molecules that will be emitted into the atmosphere each year.
Claim: To the first approximation, kA is independent of temperature.
Justification: Absorption of CO2 takes place at the surface of the ocean. The air pressure at sea-level is dictated by the weight of air per square meter pressing on the sea, which is roughly ten tonnes per square meter. When the atmosphere warms up, its mass does not change. The pressure at sea-level remains constant. But the air does expand. Because the air expands, the number of CO2 molecules per cubic meter decreases. Because the gas is warmer, each CO2 molecule is moving faster. Their average velocity is proportional to the square root of the absolute temperature. Suppose we increase the temperature from 14°C to 15°C. Absolute temperature increases from 287 K to 288 K. According to the gas law, the number of CO2 molecules per cubic meter at the ocean surface decreases by 0.35%. But their velocity increases by 0.18%, which means each CO2 molecule above the ocean surface collides with the ocean 0.18% more frequently than before. Combining these two effects, there will be a net 0.18% decrease in the number of opportunities for CO2 molecules to be absorbed. To the first approximation, this is no change at all. Let us also consider whether a faster-moving CO2 molecule, having collided with the ocean, is more or less likely to be absorbed. When CO2 dissolves in water, energy is released. The CO2 molecule does not need to supply any energy in order to be dissolved. A hotter CO2 molecule has more energy, but this energy is not required by dissolution, and will not make the reaction more likely. To the first approximation, therefore, a 1°C rise in temperature will have no significant effect upon kA.
Claim: To the first approximation, KR increases by a factor of 1.028 for each 1°C warming of the ocean surface.
Justification: Emission of CO2 takes place at the surface of the ocean. When the ocean warms by 1°C, its volume increases by 0.02%, which is insignificant. As we showed above, the warmer CO2 molecules will collide with the ocean surface 0.18% more often, but this, too, is insignificant. Let us consider whether a faster-moving CO2 molecule, having reached the surface of the ocean, is more or less likely to be emitted into the atmosphere. The emission of CO2 from solution requires heat. The enthalpy of dissolution for CO2 in water is −19.4 kJ/mole = −0.20 eV per CO2 molecule. (Compare to 0.42 eV latent heat of vaporization and −0.062 eV latent heat of fusion for an H20 molecule, and 0.26 eV latent heat of sublimation for a CO2 molecule.) Each CO2 molecule dissolving in water releases 0.20 eV of heat. Conversely, in order to be un-dissolved, a CO2 molecule needs to supply at least 0.20 eV from its own thermal energy. At 14°C, the average molecule has kinetic energy only 0.025 eV. But a tiny fraction will have, by random collisions, a much higher energy, as dictated by the Boltzmann distribution. In a given period of time, the number of molecules that attain energy E is proportional to e−E/kT, where T is absolute temperature, k = 8.62×10−5 eV/K is the Boltzmann constant, and e = 2.72 is the exponential constant. The rate at which any reaction requiring thermal energy E takes place is proportional to e−E/kT. Thus kR is proportional to e−E/kT, which means there is some constant, A, with units of 1/yr, for which kR = A e−0.2/0.0000862T = Ae−2300/T. In our natural, equilibrium carbon cycle, kR = 0.00048 1/yr. Suppose the natural, equilibrium temperature of the ocean surface is 14°C = 287 K. We can obtain the value of A as follows.
kR @ 287K = Ae−2300/287 = A×0.00033 = 0.00048 1/yr ⇒ A = 1.45 1/yr.
We raise the temperature by 1°C to 15°C = 288 K and re-calculate the rate.
kR @ 288K = 1.45 e−2300/288 = 0.000493 1/yr.
The rate has increased by a factor of 0.000493/0.00048 = 1.028, which is 2.8%. If we use 10°C or 20°C as our initial ocean temperature, we get 2.9% and 2.7% rises respectively. To the first approximation, each 1°C increase in temperature increases kR by 2.8%, regardless of our guess at the initial temperature of the ocean surface.
We simulate the effect of a sudden 1°C warming of our carbon cycle in the following way. We begin with our natural, equilibrium atmosphere, described by our carbon cycle model. We increase kR by 2.8%. We set the fossil fuel emission, mF, to zero. We obtain the following plot of atmospheric CO2 concentration versus time.
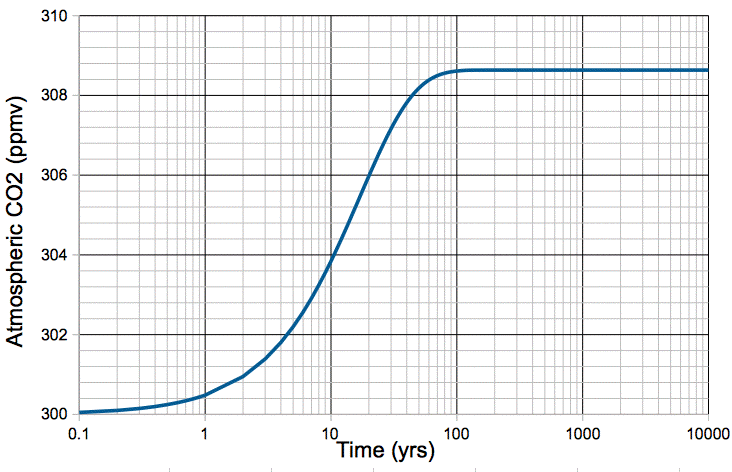
Figure: Atmospheric CO2 Concentration Following a 1°C Step Increase in Temperature.
The increase in temperature causes an immediate 2.8% increase in the rate at which CO2 is emitted by the ocean, but no increase in the rate at which it is absorbed. The additional CO2 released by the ocean builds up in the atmosphere. As the concentration of CO2 in the atmosphere increases, the rate at which it is absorbed by the ocean increases, because there are more CO2 molecules available for absorption. When the atmospheric concentration has increased by 2.8%, the rate of absorption is once again equal to the rate of emission. The carbon cycle reaches its new equilibrium in roughly one hundred years.
In our next post, we will see how well our calculations agree with the many empirical observations of carbon dioxide in solution, and with the history of our atmosphere over the past half-million years.
Claim: To the first approximation, kA is independent of temperature.
Justification: Absorption of CO2 takes place at the surface of the ocean. The air pressure at sea-level is dictated by the weight of air per square meter pressing on the sea, which is roughly ten tonnes per square meter. When the atmosphere warms up, its mass does not change. The pressure at sea-level remains constant. But the air does expand. Because the air expands, the number of CO2 molecules per cubic meter decreases. Because the gas is warmer, each CO2 molecule is moving faster. Their average velocity is proportional to the square root of the absolute temperature. Suppose we increase the temperature from 14°C to 15°C. Absolute temperature increases from 287 K to 288 K. According to the gas law, the number of CO2 molecules per cubic meter at the ocean surface decreases by 0.35%. But their velocity increases by 0.18%, which means each CO2 molecule above the ocean surface collides with the ocean 0.18% more frequently than before. Combining these two effects, there will be a net 0.18% decrease in the number of opportunities for CO2 molecules to be absorbed. To the first approximation, this is no change at all. Let us also consider whether a faster-moving CO2 molecule, having collided with the ocean, is more or less likely to be absorbed. When CO2 dissolves in water, energy is released. The CO2 molecule does not need to supply any energy in order to be dissolved. A hotter CO2 molecule has more energy, but this energy is not required by dissolution, and will not make the reaction more likely. To the first approximation, therefore, a 1°C rise in temperature will have no significant effect upon kA.
Claim: To the first approximation, KR increases by a factor of 1.028 for each 1°C warming of the ocean surface.
Justification: Emission of CO2 takes place at the surface of the ocean. When the ocean warms by 1°C, its volume increases by 0.02%, which is insignificant. As we showed above, the warmer CO2 molecules will collide with the ocean surface 0.18% more often, but this, too, is insignificant. Let us consider whether a faster-moving CO2 molecule, having reached the surface of the ocean, is more or less likely to be emitted into the atmosphere. The emission of CO2 from solution requires heat. The enthalpy of dissolution for CO2 in water is −19.4 kJ/mole = −0.20 eV per CO2 molecule. (Compare to 0.42 eV latent heat of vaporization and −0.062 eV latent heat of fusion for an H20 molecule, and 0.26 eV latent heat of sublimation for a CO2 molecule.) Each CO2 molecule dissolving in water releases 0.20 eV of heat. Conversely, in order to be un-dissolved, a CO2 molecule needs to supply at least 0.20 eV from its own thermal energy. At 14°C, the average molecule has kinetic energy only 0.025 eV. But a tiny fraction will have, by random collisions, a much higher energy, as dictated by the Boltzmann distribution. In a given period of time, the number of molecules that attain energy E is proportional to e−E/kT, where T is absolute temperature, k = 8.62×10−5 eV/K is the Boltzmann constant, and e = 2.72 is the exponential constant. The rate at which any reaction requiring thermal energy E takes place is proportional to e−E/kT. Thus kR is proportional to e−E/kT, which means there is some constant, A, with units of 1/yr, for which kR = A e−0.2/0.0000862T = Ae−2300/T. In our natural, equilibrium carbon cycle, kR = 0.00048 1/yr. Suppose the natural, equilibrium temperature of the ocean surface is 14°C = 287 K. We can obtain the value of A as follows.
kR @ 287K = Ae−2300/287 = A×0.00033 = 0.00048 1/yr ⇒ A = 1.45 1/yr.
We raise the temperature by 1°C to 15°C = 288 K and re-calculate the rate.
kR @ 288K = 1.45 e−2300/288 = 0.000493 1/yr.
The rate has increased by a factor of 0.000493/0.00048 = 1.028, which is 2.8%. If we use 10°C or 20°C as our initial ocean temperature, we get 2.9% and 2.7% rises respectively. To the first approximation, each 1°C increase in temperature increases kR by 2.8%, regardless of our guess at the initial temperature of the ocean surface.
We simulate the effect of a sudden 1°C warming of our carbon cycle in the following way. We begin with our natural, equilibrium atmosphere, described by our carbon cycle model. We increase kR by 2.8%. We set the fossil fuel emission, mF, to zero. We obtain the following plot of atmospheric CO2 concentration versus time.

Figure: Atmospheric CO2 Concentration Following a 1°C Step Increase in Temperature.
The increase in temperature causes an immediate 2.8% increase in the rate at which CO2 is emitted by the ocean, but no increase in the rate at which it is absorbed. The additional CO2 released by the ocean builds up in the atmosphere. As the concentration of CO2 in the atmosphere increases, the rate at which it is absorbed by the ocean increases, because there are more CO2 molecules available for absorption. When the atmospheric concentration has increased by 2.8%, the rate of absorption is once again equal to the rate of emission. The carbon cycle reaches its new equilibrium in roughly one hundred years.
In our next post, we will see how well our calculations agree with the many empirical observations of carbon dioxide in solution, and with the history of our atmosphere over the past half-million years.
Subscribe to:
Posts (Atom)
