We run the simulation starting with our cold-start state, CS_0hr. The program runs ten times slower than before. The water balancing calculations are more complex now that we have added precipitation, and we must perform them more often because rain and snow move quickly through the atmosphere. Nevertheless, a few hours running gives us six weeks of simulation time, and the atmosphere converges to the equilibrium state shown below, which you will find stored in SR_1200hr. The light gray cells are clouds of water droplets. The white cells are clouds of snowflakes. The dark gray cells are rain.
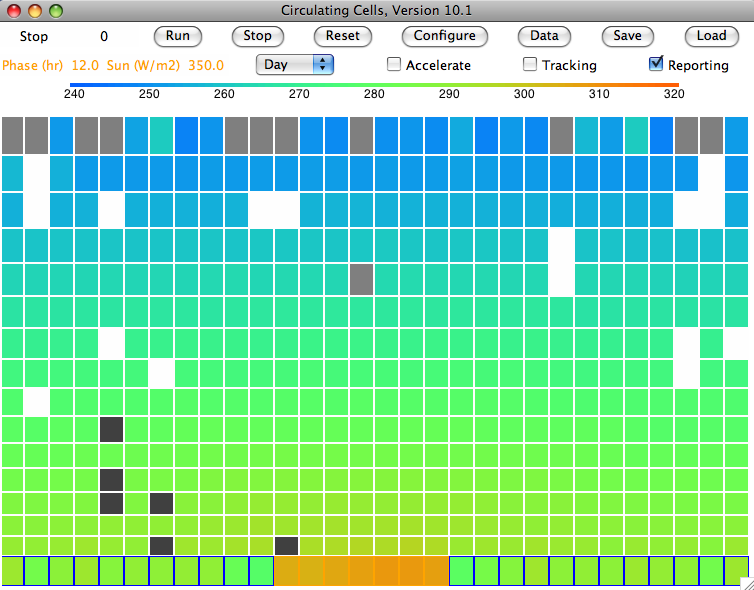
We see clouds of water droplets in the top row of cells. Here they are cooling by radiation. Our atmosphere is still opaque to long-wave radiation (transparency fraction is zero). Only the top cells can radiate into space. Their temperature is, however, well below 268 K (Tf_droplets), the temperature at which droplets are transformed into ice crystals. Snow forms within the clouds at 0.001 g/kg/s (freeze_rate_gps) and falls at 1 m/s (snow_speed_mps). When it sinks through a cell warmer than 278 K (Tm_ice), it melts at 0.01 g/kg/s (melt_rate_gps), forming rain. Rain falls at 5 m/s (rain_speed_mps).
The following graph shows how surface air temperature and average cloud depth vary with time from our cold start. The final cloud depth fluctuates by ±0.5 mm around an average value of 1.7 mm. The average temperature of the surface gas is 292 K, which is 19°C. Of the light that arrives from the Sun, 30% is reflected into space, giving our simulated planet an albedo of 0.3, which matches that of our own planet Earth.
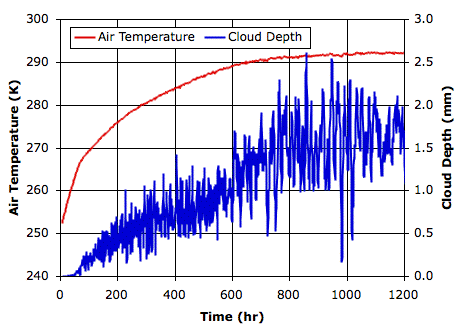
With fast-sinking clouds, the cloud depth remained close to 2.9 mm and the surface temperature was −7°C. Evaporation from water at 19°C is roughly ten times faster than from from water at −7°C, but precipitation is so effective at removing water from the atmosphere, the sky is almost entirely clear. Indeed, the Sun shines directly upon our island half the time, heating its sandy surface up to 34°C.

I have downloaded CC10 and I’m reading it. In your foreword I read:
ReplyDelete“The pressure just above a cell is 1 kPa greater than the pressure just below it.”
Really, it may just be a typing error. I will read the rest with the needed attention.
I wish you all the best for the next new year.
Michele
That is a typing error. It should read "The pressure just BELOW a cell is 1 kPa greater."
ReplyDeleteIt's such a long time since we had watched your code and I encounter very much difficulties in reading it. I have a question for you.
ReplyDeleteWhy do you assume that the water vapour is continuously operating above the triple point?
Michele
I suppose you could say I'm assuming no triple point. But the transfer of liquid water to ice is the sort of thing that occurs when the three states co-exist. I could do better with the co-existence, but I have to come up with some calculations that capture the most important features of rain-formation, and I think I have done that.
ReplyDeleteHi Kevan,
ReplyDeleteI find it a little strange that your blog is no longer updated, it was for me a pleasant habit.
I hope that this interruption is due to your effort and nothing else. I will do my very best wishes to you and greet you with warmth.
Michele
Dear Michele,
ReplyDeleteGreat to hear from you. I have been updating every week, and this week, arrived at the conclusion of the simulation efforts, with a post on Anthropogenic Global Warming. Perhaps your bookmark takes you always to this particular post. Try clicking "newer post" below, or go to the blog home page:
http://homeclimateanalysis.blogspot.com/
If you still get this page, try pressing refresh on the browser. Maybe your browser keeps giving you the same page.
Hoping to hear from you, and very glad you are still out there.
Yours, Kevan