We start with a block of four cells that has no tendency to circulate, warm up the lower-left cell, and consider the buoyancy work this single warm cell can generate for us. Here is the diagram we presented in our Buoyancy post. The cell on the lower left is warmer by δT than the adiabatic profile we derived earlier. We use R for the specific gas constant, Cv for the specific heat capacity at constant volume, and Cp for the specific heat capacity at constant pressure. As always, we have Cp=R+Cv for an ideal gas.
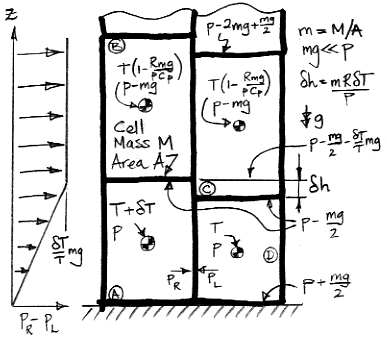
When a cell expands adiabatically from p to p−mg, its temperature drops from T to T(1−mg/p)R/Cp. When mg≪p, this expression simplifies to T(1−Rmg/pCp), which is why the upper cells in our diagram have temperature T(1−Rmg/pCp).
As we heat cell A by δT, we raise its center of mass by δh/2 and the center of mass of cell B by δh. We also raise the gas above B by δh. Cells C and D do not move, nor does the gas above them. The weight of each cell is m per unit base area, and the weight of the gas above cell B is the pressure in cell B, which is p−mg. As we supply heat to A to warm it up, the work done by the expanding cell against gravity is W1.
W1 = mgδh + mgδh/2 + (p−mg)δh = mRδT(1 + mg/2p)
We now allow the four cells to rotate one step clockwise. Cells C and D move to the bottom. They are at temperature T. Cell B will be on the upper-right, with its temperature unchanged at T(1−Rmg/pCp). Cell A, having expanded adiabatically from pressure p to p−mg, will cool down, but will still be warmer than cell B by δT ' = δT(1−Rmg/pCp).
Suppose we remove heat from cell A until it is the same temperature as B. Once it has cooled by δT ', the block of four cells will be identical to the one we started with before we heated A by δT. As A cools by δT ', its center of mass drops by δh'.
δh' = mRδT '/(p−mg) = mRδT(1−Rmg/pCp)/(p−mg)
We note that mg≪p and this allows us to simplify the above expression.
δh' ≈ mRδT(1 + mgCv/pCp)/p
As cell A shrinks, its own center of mass drops by δh'/2 and the gas above drops by δh'. Now we can calculate the work done by gravity, W2, as the cell cools.
W2 = mgδh'/2 + (p−mg)δh' = δh'(p−mg/2) = mRδT(1 + mgCv/pCp)(1−mg/2p)
Once again we note that mg≪p, which allows us to simplify our expression.
W2 ≈ mRδT(1 + mgCv/pCp − mg/2p)
When we heated cell A by δT, we increased the gravitational potential energy of the cells and upper gas by and amount W1. When we allowed the cells to rotate, they produced buoyancy work, the size of which we would like to determine. When we allowed cell A to cool, we decreased the gravitational potential energy of the cells and upper gas by an amount W2, and in doing so we returned these gases to their original state. By conservation of energy, the difference between W1 and W2 must be equal to the buoyancy work.
W1−W2 = mRδT(Rmg/pCp)
The buoyancy work per kilogram of gas in the four cells, Wb, is therefore:
Wb = mgR2δT/4pCp
We interpret our expression for Wb as follows. When the cells rotate, cell B drops by δh, which does work mgδh = mgRδT/p. But at the same time, all the cells above A rise by δh'−δh>0, and this requires work mgRCvδT/pCp. The buoyancy work is the work done by B as it drops, minus the work required to raise the upper gas, divided by the mass of the four cells combined, which gives us the expression for Wb above. In our CC5 calculation, we ignored the work required to raise the upper gas, and so over-estimated the buoyancy work by a factor of Cp/R, which is 3.5 for air.
If cell B contains 300 kg/m2 of air at 100 kPa and 300 K, and δT for cell A is 10°C, the buoyancy work is 6.2 J/kg, which is sufficient to accelerate the cells to 3.5 m/s. These cells would be 250 m high, so at 3.5 m/s they would circulate in one minute. Using our old calculation of buoyancy work, the circulation would take almost twice as long.

Let’s consider a tank of water and an elastic heavy ball inside it filled whit air and in equilibrium. We have the situation at left side of ”(this figure)” and the surface of separation air/liquid is at the height H from the bottom of the tank. If we heat the ball the warmer inner air will expand spherically as the pressure is isotropic and the surface air/liquid will rise δH. So at any point inside the tank the pressure will increase &ro;gδH. The balance of the forces no longer exists, and the ball moves upwards, the inside/outside pressures decrease, the inner air expands, the height of the surface air/liquid increase, i.e., the air of the ball works on the liquid which increases its geo-potential energy. All this is based on the perfect impermeability of the ball to the air and the liquid.
ReplyDeleteThe walls of your cells are fictitious and the cell, if alone, suddenly vanishes. A phalanx of cells have more possibilities in prosecuting its existence sacrificing the extreme cells.
Michele
I assume the density of the ball is greater than that of water, so that the combined density of the ball and air is equal to that of the fluid. So, I see that the ball will be at equilibrium at the start. When we heat the air, I agree that the expanding gas does work upon the fluid to raise its surface. I agree that as the ball rises, it will expand more. But this further expansion is not necessary for it to rise. If we started with a cell of oil in water, the oil is held together only by surface tension, and it does not expand as it rises, nor does it beak apart, and yet it rises.
ReplyDeleteYours, Kevan