In Water Vapor Continuum we found that a simple scaling of absorption with concentration is grossly inaccurate for water vapor. In Earth Radiator we showed that a 1-mm layer of water absorbs over 99.9% of all long-wave radiation. The graphs we present in Continuum Absorption Length imply that the same amount of water dispersed in a 1-km column of air will absorb less than 10% of long-wave radiation.
It turns out that a simple scaling calculation does not apply to CO2 either. If the absorption length of 20-μm radiation were 3 km in air with 330 ppm CO2, the Earth's atmosphere would be opaque to 20-μm radiation. But according to this popular graph, the CO2 in the Earth's atmosphere is transparent to 20 μm radiation.
In Absorption Line Broadening in the Infrared by Burch et al, we see how the presence of nearby molecules of all gases broadens the sharp absorption lines of the CO2 molecule. With high enough pressure, these lines become so broad that they overlap, and we see absorption of long-wave radiation at all frequencies within certain ranges, such as 13 μm to 17 μm.
We concluded that calculating the absorption spectra of water vapor and CO2 was far too big a project for us to embark upon. So we paid for a month's subscription to the most excellent Spectral Calculator. We asked the calculator to plot absorption by dry air with 330 ppm CO2 at 300 K and 1 atmosphere over a path length of 1 km. The calculator produced the following graph. For a logarithmic plot of the same data see here.
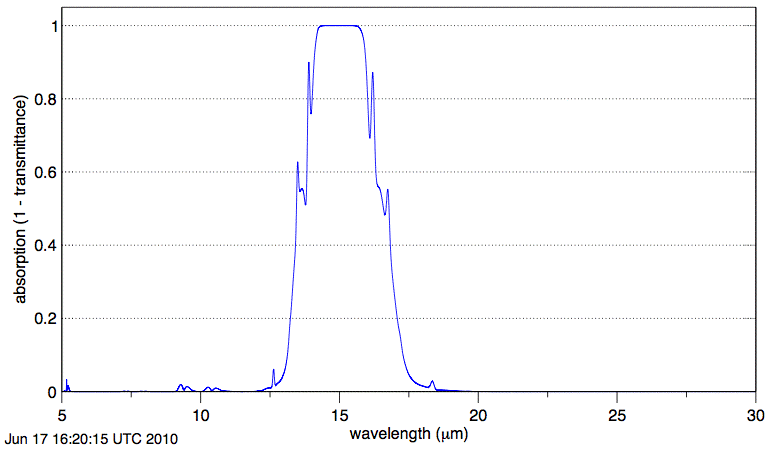
Absorption by CO2 along our 1-km path is negligible at wavelength 20 μm. The graph shows us that absorption of long-wave radiation by atmospheric CO2 is significant only in the range 12.5 μm to 17.5 μm.

No comments:
Post a Comment