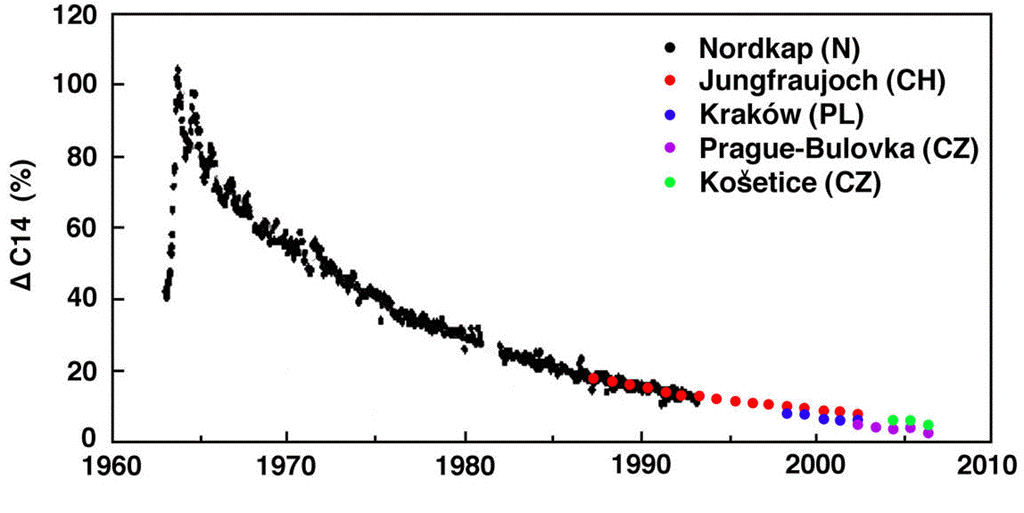
Figure: Atmospheric Concentration of Carbon-14 During and After the Bomb Tests. This graph is from an essay by Pettersson. The author combined measurements from several stations to produce the most complete graph we could find. For alternate plots, see here and here.
The relaxation of the atmospheric carbon-14 concentration from its peak follows an exponential decay with time constant roughly 15 years. After fifty years, the concentration is within a few percent of its value before the bomb tests. The carbon-14 did not linger in the atmosphere. It disappeared. Let's see if our model of the Earth's carbon cycle predicts this same relaxation or not.
In our model, carbon-14 concentrations are governed by two differential equations. We already solved these equations for a starting-point of zero in the atmosphere and the reservoir. Using the same procedure, we can solve the equations for a starting point of 2.0 ppt in the atmosphere and 0.8 ppt in the reservoir, which is the state the atmosphere was in at the time of the Partial Test Ban Treaty, when atmospheric tests were stopped. We obtain the following solutions for atmospheric concentration, CA, and reservoir concentration CR.
CA = 1.0 + 0.987 e−t/17 + 0.013 e−t/8200
CR = 0.8 + 0.0089 e−t/17 − 0.0089 e−t/8200
We note that this as t → ∞, we have CA → 1.0 ppt and CR → 0.8 ppt, as before. Whatever carbon-14 we add to the atmosphere, it will eventually decay, and we will be left once again with the concentrations generated by cosmic ray production. The above equations produce the graph of atmospheric and reservoir concentration shown below.
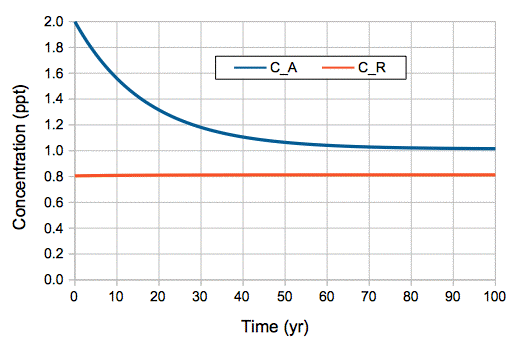
Figure: Carbon Cycle Model's Prediction of Atmospheric and Reservoir Carbon-14 Concentration After Sudden Doubling of Atmospheric Concentration.
Our simple, two-part, uniform-reservoir model of the carbon cycle predicts an exponential relaxation of carbon-14 concentration after the bomb tests, with a time constant of 17 years. Needless to say: agreement between the model and observation is superb.

No comments:
Post a Comment