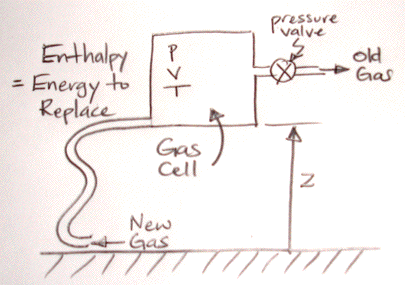
New gas entering at the lower-left corner of the cell must be at pressure p in order to force the old gas out of the pressure valve on the upper-right. Suppose the cross-section of the pipe is A and we push the gas in with a piston. The piston must move a total distance V/A to replace all the old gas. The front face of the piston must push with force pA. The work we do is pA×V/A = pV. The product pV is the pressure energy that we have mentioned before, but never explained in detail.
If we push the gas into the pipe at ground level, we have to do extra work to raise all the replacement gas up to the altitude of the cell. If the mass of the cell is m and gravity is g, the work we must do to raise the new gas is mgz. This is the gravitational energy component of enthalpy. When we use ρ for density, the gravitational energy becomes Vgzρ.
Our new gas must be at the same temperature as the old. We could choose 300 K as the temperature of the gas in our hypothetical reservoir. But we choose 0 K instead, because this eliminates a constant in our equations. The constant is of no use to us because we find that we are interested only in changes in enthalpy, never the absolute value of enthalpy.
Suppose our gas is ideal, with specific heat capacity at constant volume Cv. The heat required to warm up our replacement gas is mTCv. If we use Vρ for m the heat is TVρCv. The total energy required to replace the old gas in our cell with new gas from our hypothetical reservoir is the sum of the heat, gravitational energy, and pressure energy.
Enthalpy = H = pV + Vgzρ + TVρCv
The specific enthalpy is the enthalpy per kilogram. We divide H by the mass Vρ to obtain the following, in which we use V to denote the volume per kilogram, which is the same as 1/ρ.
Specific Enthalpy = h = pV + gz + CvT
For an ideal gas we have pV = RT, where R is the specific gas constant. We are already familiar with the heat capacity at constant pressure, Cp = R + Cv, so we arrive at the following expression for the specific enthalpy of an ideal gas.
h = (R + Cv)T + gz = CpT + gz
When we establish convection in an atmosphere, the variation in temperature with altitude must be such that no work is required to move volumes of gas up and down. We established this principle in a previous post and showed with a dozen lines of calculations that the variation of temperature with altitude must be linear with slope −g/Cp. Now let us make the same point using the concept of specific enthalpy.
The enthalpy of a kilogram of gas is the energy required to replace it from our hypothetical infinite reservoir. The energy required to move a kilogram of gas from one place to another in our atmosphere is the difference in its enthalpy at the two locations. Our assumption that no work is required to move gas up and down means that the specific enthalpy of the gas in the atmosphere must be constant with altitude.
dh/dz = CpdT/dz + g = 0 ⇒ dT/dz = −g/Cp
And so we see that the concept of enthalpy allows us to deduce the dry adiabatic lapse rate in two lines instead of twelve. Enthalpy is a useful tool for calculations. But we note that the pressure and internal energy calculation we performed in our previous post is the one that shows us the physical mechanisms by which enthalpy is conserved, and is therefore important for our understanding.
We will use enthalpy in our next post to help solve the mystery of why expansion work and buoyancy work are equal in our circulating cells simulation.

Be careful, Kevan,
ReplyDeletethe gravitational energy is a form of the energy different than the thermal one!
Whatever body (solid, liquid) having the mass M undergoes the variation MgΔz of its own potential energy without its temperature changes.
The enthalpy of a gas is a function of its thermodynamic state and is defined as h = CvT + pv and we can consider it as the total thermal-elastic energy of the gas.
For the atmospheric process we have to refer to the total specific energy of the gas which is affected by the movement with strong differences of height, i.e., (h + gz) that is constant in absence of work done on/by the gas.
Michele
Dear Michele,
ReplyDeleteThank you for your comment, and I think I understand your point. I delayed this post a week so I could think about it from many different sides (and my wife had a baby too, all is well). Your point about liquid rising was one I spent many hours thinking about, as you can see in my previous post about expansion and buoyancy work.
When we look at the forces acting upon the cell, we see that the weight of other cells acts only on the boundaries. The cell has weight that is counter-acted by the pressure difference from bottom to top, which is always constant for slow-moving cells. Other than this pressure difference, the weight has no affect upon the cell. The weight of other cells appear as pressure on top and bottom. Furthermore, the weight of other cells is the only source of pressure.
Any calculation that accounts for pressure on the cell is one and the same as a calculation that accounts for gravitational energy. The energy calculation and the pressure calculation produce the same answer because they are the same phenomenon.
In a gas is that the temperature and pressure energy are proportional to one another, so that they must change at the same time. Thus it is not possible for a gas to rise up without cooling, but it is possible for a body of liquid to do so.
Yours, Kevan
Kevan,
ReplyDeletemy best wishes for your new child. I hope that everything has been all right.
Michele