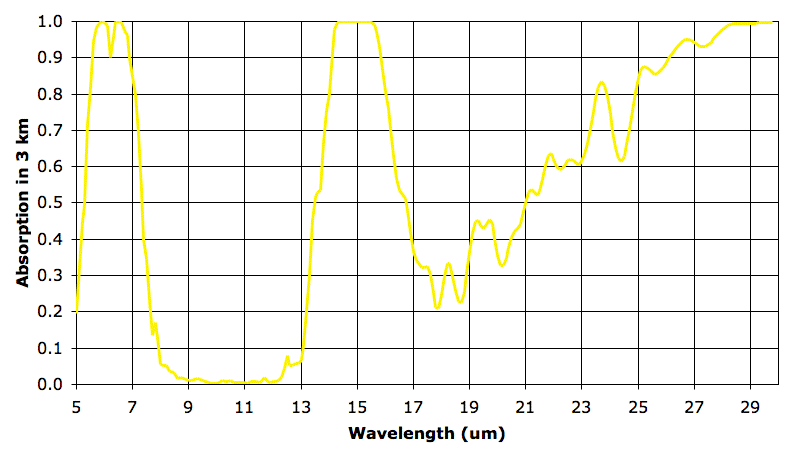
As before, we say our 3-km layer is transparent at a particular wavelength when it absorbs less than 63% of that wavelength. The third 3-km layer is transparent from 5.0 to 5.4 μm, 7.3 to 13.8 μm, and 16.3 to 23.1 μm.
The transparency in the range 16.3 to 23.1 μm is due to the drop in water vapor concentration and pressure that occurs as we ascend through the atmosphere. Absorption in this band is due mostly to water vapor dimers instead of bonds within the water molecule. Absorption by dimers is proportional to the square of the pressure. Continued absorption in 5.4 to 7.3 μm is due to bonds within water vapor molecules, and does not drop with pressure as quickly as absorption by dimers. Continued absorption in the range 13.8 to 16.3 μm range is mostly due to CO2.

No comments:
Post a Comment