Our calculations so far go like this. Every year, cosmic rays create 7.5 kg of carbon-14 from atmospheric nitrogen. Carbon-14 decays back into nitrogen with a half-life of 5700 years, which means 0.012% of it decays into nitrogen every year. After fifty thousand years, the total mass of carbon-14 in the atmosphere will reach equilibrium. The total mass of carbon-14 will be 62,500 kg, because 62,500 kg multiplied by 0.012% is 7.5 kg per year, so the rate at which carbon-14 is created by cosmic rays is equal to the rate at which the Earth's reservoir of carbon-14 decays back into nitrogen.
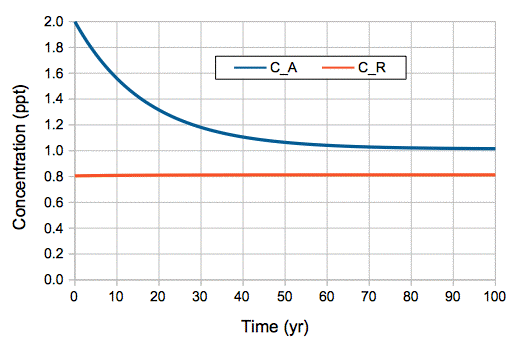
The natural, equilibrium atmosphere of the early twentieth century contained 650 kg of carbon-14. The remainder of the Earth's 62,500 kg of carbon-14 is elsewhere, in the reservoir of our carbon cycle. Almost all carbon-14 in the atmosphere exists in CO2. By whatever means a carbon-14 atom moves in and out of the atmosphere, it does so in CO2 molecules. When one carbon-14 molecule moves into the reservoir, it does so in the company of 1÷1.0 ppt = 1.0 trillion CO2 molecules. If the reservoir is the deep ocean, where the concentration of carbon-14 is 0.8 ppt, we can further say that, whenever a carbon-14 atom re-enters the atmosphere from the ocean, it does so in the company of 1÷0.8 ppt = 1.25 trillion CO2 molecules.
We calculated that the reservoir, assuming it is the deep ocean, must contain 77,000 Pg of carbon, and that every year 37 Pg of carbon must be exchanged between the reservoir and the atmosphere, all of it moving with CO2 molecules. When 37 Pg of carbon moves from the atmosphere to the reservoir, it carries with it 37.0 kg of carbon-14. When 37 Pg of carbon moves from the reservoir to the atmosphere, it carries with it 29.6 kg of carbon-14. The difference is a net 7.4 kg of carbon-14 flowing into the reservoir every year. We add to this another 0.1 kg per year, which is the decay of the carbon-14 that remains in the atmosphere, and we arrive at a total of 7.5 kg of carbon-14 being removed from the atmosphere by exchange and decay, which gives us equilibrium with the 7.5 kg per year being created by cosmic rays.
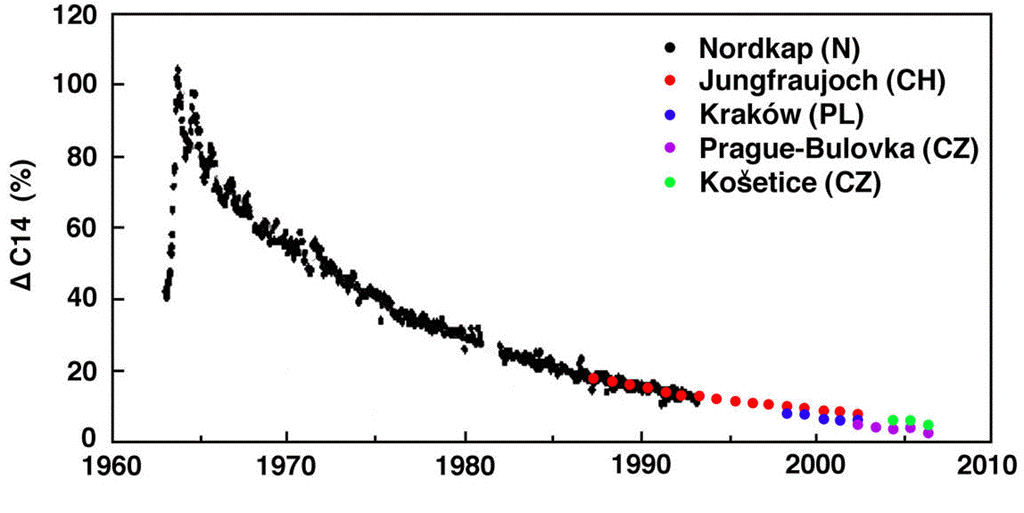
With the above values of reservoir size and annual mass exchange, we obtained an analytic solution to the carbon-14 concentration in our natural, equilibrium atmosphere. We showed that this analytic solution was in near-perfect agreement with the relaxation-time of atmospheric carbon-14 concentration following the nuclear bomb tests of the 1950s and 1960s. Thus we are confident that our analytic solution is a reliable model for predicting the behavior of carbon-14, and therefore of all carbon, in the Earth's atmosphere.
Let us consider the possibility that the reservoir of our carbon cycle resides somewhere other than the deep ocean. The table below shows other candidates for the reservoir, their carbon-14 concentrations, and the relaxation-time of carbon-14 concentration that we would obtain if this candidate were indeed the carbon-14 reservoir. We refer to Arnold et al. for our normalized concentration values, in which the atmospheric concentration is taken to be 1.0 ppt.
| Candidate | Carbon-14 Concentration (ppt) | Relaxation Time (yr) |
|---|---|---|
| Ocean, Below 1000 m | 0.80 | 17 |
| Ocean, Top 100 m | 0.96 | 3.5 |
| Biosphere, Land | 1.00 | 0.0 |
| Biosphere, Ocean | 0.96 | 3.5 |
| Soil, Humus | 1.00 | 0.0 |
The above candidate reservoirs are the only ones known to us that exchange CO2 with the atmosphere. The relaxation-time of carbon-14 concentration after the nuclear bomb tests was roughly 15 years. None of the above candidates are even close to being consistent with the aftermath of the bomb tests, except for the deep ocean, which is in excellent agreement. We conclude that the reservoir of our carbon cycle is the deep ocean and only the deep ocean. The other candidate reservoirs do exchange CO2 with the atmosphere, but whatever effect they have upon the Earth's carbon cycle is dwarfed by the flow of carbon into and out of the deep ocean.