The table below presents the thermal properties of some common surface materials. We will make use of this table in future posts. Values for composite materials like wet soil are approximate. We picked values that are representative from resources like this, this, and this. Today we will use only the properties of dry sand. In the first column we have the thermal conductivity in Watt per meter per Kelvin (W/mK = W/m°C). A layer of material with thermal conductivity α, thickness d, temperature Tb at the bottom and Tt at the top, will conduct α(Tb−Tt)/d for each square meter of its surface area. A 10-cm thick layer of dry sand that is 20°C cooler on top than bottom will conduct 0.20 W/mK × 20 °C / 0.1 m = 40 W/m2.
Conductivity Density CapacityThe second column gives the density in kilogram per cubic meter (kg/m3). A 10-cm layer of dry sand has mass 0.1 m × 1700 kg = 170 kg/m2. The third column gives the specific heat capacity in Joule per kilogram per kelvin ( J/kgK = J/kg°C). A layer of material with specific heat capacity C, density ρ, and thickness d will require Cρd Joules of heat per square meter of surface area to raise its temperature by 1°C. A 10-cm thick layer of dry sand requires 800 J/kgK × 1700 kg × 0.1 m = 136 kJ/m2 to warm up by 1°C. Alternatively, we must remove 136 kJ/m2 to cool it by 1°C. If this layer loses loses 80 W/m2, it will take half an hour to cool down by 1°C.
Material (W/mK) (kg/m^3) (J/kgK)
Air 0.024 1.2 1000
Asphalt 0.75 1300 920
Copper 390 8900 420
Ice 2.2 910 2000
Mercury 8.7 14000 140
Sand, Dry 0.20 1600 830
Sand, Wet 4.0 2000 2500
Sandstone 1.7 2500 800
Soil, Dry 0.4 1300 1400
Soil, Wet 4.0 1500 2500
Snow, Light 0.10 100 420
Steel 43 7800 460
Water 0.58 1000 4200
Wood, Oak 0.17 770 1700
Our SC1 program simulates the cooling of the surface of the Earth at night. You can download it and run it yourself, by following the instructions in the code. The program works by dividing the surface material into thin layers and calculating the temperature of each layer in a sequence of small time steps. We assume the material is at a uniform temperature when it begins to cool, and that the heat loss from the upper surface is a constant 200 W/2. In earlier versions of the code, we calculated the surface loss as a combination of radiation into space and convection with the surface air, but our results were pretty much the same, so we reverted to the assumption of constant loss to keep things simple.
The following graph shows what our simulation program tells us happens to dry sand at various depths in millimeters when it starts to cool from the surface at 200 W/m2. We see the surface cools by 13°C in 1000s while the sand 40 mm down cools by roughly 0.1°C.
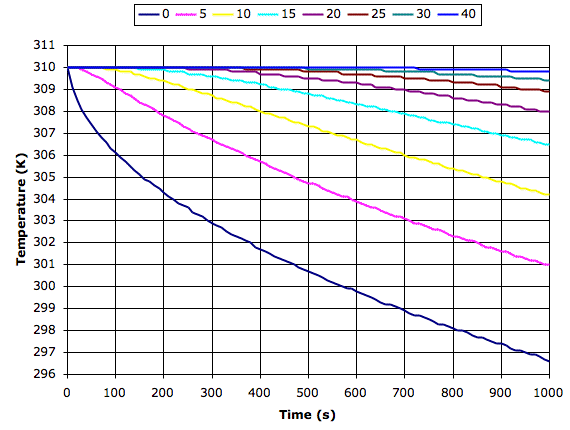
Materials with greater conductivity cool more slowly because heat rises from below to keep the surface warm. If we apply our simulation program to sandstone or asphalt, we find the surface cools down ten times more slowly than the surface of dry sand. So it is above dry sand or soil that we expect to see the greatest drop in surface temperature at night.
Our calculations show that a sandy surface can cool by 20°C at night. They show that one meter down, the heat of the day will hardly affect the temperature of the sand. But they still don't explain why the temperature above the desert sand cools by 20°C at night. The surface of the sand cools down because it loses heat to the air and to outer space. If anything, the cooling sand warms the air. We already know that the heat radiated by the air itself is not sufficient to cause such cooling. Indeed, our calculations show that it is impossible for air above the desert to cool by 20°C at night. And yet we know that a thermometer 2 m above the surface of the desert can register a drop of 20°C.
We conclude that the air above the sand at night cannot be the same air above the sand during the day, and indeed this conclusion leads us to a most satisfactory explanation for cold desert nights.

Dear Kevan,
ReplyDeletejust to underline a little misprint. You wrote "The third column ... Joule for kilogram for kelvin (J/kgK = W/kg°C)". Naturally it’s "J/kg°C".
Your writing is as a trilling; I like it.
Regards, Michele
Dear Kevan,
ReplyDeleteLet me go off the post subject.
I have found this presentation at
www.asic3.sdl.usu.edu/papers/mlynczak.ppt
whose slide number 11 put in evidence that the brightness temperatures are not affected from the effective size of clouds particles.
Is it true and what this can have to do with that you affirmed about the IR clouds absorption?
Regards, Michele
Dear Michele,
ReplyDeleteThank you for the correction.
I used your link to the mlynczak paper and obtained a .ppt.pps file that I can't open. I'm not sure what you mean by "brightness temperature."
The IR absorption of a cloud is not a function of the size of the droplets, but the volume of water per square meter of cloud surface. If the cloud contains 1 liter of water per square meter, this is equivalent to 1 mm depth of water distributed over one square meter. This 1 mm is absolutely black to IR. If the cloud contains only 10 ml/m^2, the equivalent water depth would be only 10 um. Even at 10 um, water absorbs most IR, as you can see here:
http://homeclimateanalysis.blogspot.com/2010/01/earth-radiator.html
Yours, Kevan
Hi Kevan,
ReplyDeleteAnother minor typo: in your red table the units in the conductivity column should presumably be W/mK not W/m2.
Cheers,
Hugh
Dear Hugh, You are indeed correct. Thank you. Kevan
ReplyDeleteDear Michel,
ReplyDeleteI think I understand the slide you sent me about cloud particle size. It appears that the clouds are at the same temperature, but the ice particles (you pointed out in an earlier comment that the particles will be ice in cirrus clouds) are off different sizes. The radiation remains unaffected. This is consistent with ice being opaque to all IR wavelengths.
Another slide you sent me, about clear sky cooling, was very interesting, and sent me looking at a bunch of slides in several places, talking about clear sky cooling. Their study of the absorption and radiation in the atmosphere did not lead to solid conclusions, so I felt that the complexity of their arguments, although impressive, was not something I would go into here. Here we have a simple, layered approach that gives the right answer for CO2 sensitivity. Our discussion talks about Total Escaping Power, and is easy to adjust for clouds. So I'm not going to start talking about clear-sky cooling.
Thank you again for your slides, corrections, and questions.
Yours, Kevan